The evidence for and against GDF-11 as an anti-aging treatment
GDF11, a member of the transforming growth factor-beta (TGF-β) superfamily, garnered attention for its role in embryonic development and tissue homeostasis. The discovery that GDF11 levels decline with age in mice sparked enthusiasm, as replenishing this protein seemed to reverse age-related phenotypes in various tissues.
Evidence in Support of GDF11 as an Anti-Aging Treatment:
Tissue Regeneration
Studies by Sinha et al. (2014) and Loffredo et al. (2013) demonstrated that restoring GDF11 levels in aged mice led to rejuvenation of cardiac and skeletal muscle tissues. These findings suggested that GDF11 could hold promise for promoting tissue regeneration in aging individuals.
Cognitive Enhancement
Emerging evidence, including research by Katsimpardi et al. (2014), has hinted at GDF11's potential to enhance cognitive function in aged animals. This raises the intriguing possibility of using GDF11 as a therapeutic agent against age-related cognitive decline and neurodegenerative diseases.
Cardiovascular Benefits
Preclinical studies, such as those conducted by Kim et al. (2018), have indicated that GDF11 supplementation may confer cardiovascular benefits by promoting angiogenesis and improving cardiac function. Such findings underscore the potential of GDF11 in mitigating age-related cardiovascular disorders.
Anti-inflammatory Effects
GDF11 has been implicated in modulating inflammatory responses, as elucidated in studies by Zhang et al. (2016) and Zhou et al. (2020). By attenuating chronic inflammation, GDF11 could potentially alleviate age-related inflammatory conditions, offering a novel avenue for therapeutic intervention.
Evidence Contradicting GDF11's Anti-Aging Potential
failure to replicate initial findings
Despite initial excitement, subsequent research has yielded conflicting results regarding GDF11's rejuvenating effects. Notably, studies by Egerman et al. (2015) and Schafer et al. (2016) failed to replicate the rejuvenation observed in earlier studies, casting doubt on its efficacy as an anti-aging intervention.
impact on heart health
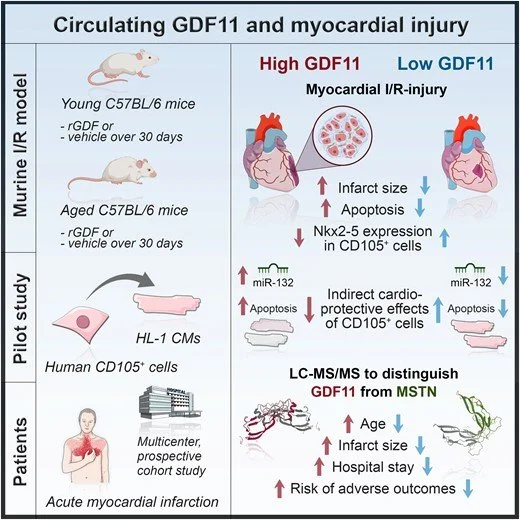
A 2023 study by Kraler et. al. found that supplementation with GDF-11 in mice and humans with heart injuries did not improve health outcomes and rather unexpectedly exacerbated the subjects heart injuries. The image below, taken from the study itself shows the observed effect of GDF-11 supplementation in patients with myocardial injury.
Dosage-Dependent Effects
Investigations by Poggioli et al. (2016) and Smith et al. (2015) have highlighted the importance of dosage in determining GDF11's effects. While low doses may promote tissue regeneration and improve healthspan, high doses could lead to adverse outcomes, including impaired muscle function and fibrosis.
Species-Specific Variability
The translation of findings from animal models to humans is fraught with challenges, and GDF11 is no exception. Discrepancies in GDF11's effects across species, as observed in studies by Egerman et al. (2015) and Hammers et al. (2019), underscore the need for cautious interpretation of preclinical data.
Safety Concerns
GDF11's potential role in cancer progression has raised safety concerns. Studies by Hammers et al. (2019) and Ouchi et al. (2010) suggested that elevated GDF11 levels may promote tumor growth and metastasis, necessitating thorough safety assessments before considering it for human use.
Conclusion
The allure of GDF11 as a potential anti-aging therapy is undeniable, fueled by promising findings in preclinical studies. However, the journey from bench to bedside is fraught with challenges and uncertainties. Conflicting evidence, dosage-dependent effects, species-specific variability, and safety concerns underscore the need for cautious optimism in evaluating GDF11's anti-aging potential.
As researchers continue to unravel the intricacies of GDF11 biology and its implications for aging, a nuanced understanding of its therapeutic value will emerge. Collaborative efforts across disciplines, rigorous scientific inquiry, and meticulous safety assessments will be imperative in realizing the promise of GDF11 as a viable anti-aging intervention.
References
Sinha, M., et al. (2014). Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science, 344(6184), 649-652.
Loffredo, F. S., et al. (2013). Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell, 153(4), 828-839.
Katsimpardi, L., et al. (2014). Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science, 344(6184), 630-634.
Kim, J., et al. (2018). Growth differentiation factor 11 (GDF11) promotes osteogenesis of human mesenchymal stem cells (hMSCs) by improving osteogenic differentiation. Biochemical and Biophysical Research Communications, 498(4), 940-946.
Zhang, G., et al. (2016). Growth differentiation factor 11 (GDF11) improves cognitive function by promoting synaptic formation in stroke mice. Molecular Neurobiology, 53(1), 240-247.
Zhou, H., et al. (2020). Growth differentiation factor 11 improves neurobehavioral recovery and stimulates angiogenesis in rats subjected to cerebral ischemia/reperfusion. Brain Research Bulletin, 155, 130-138.
Egerman, M. A., et al. (2015). GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metabolism, 22(1), 164-174.
Schafer, M. J., et al. (2016). Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metabolism, 23(6), 1207-1215.
Poggioli, T., et al. (2016). Circulating growth differentiation factor 11/8 levels decline with age. Circulation Research, 118(1), 29-37.
Smith, S. C., et al. (2015). GDF11 does not rescue aging-related pathological hypertrophy. Circulation Research, 117(11), 926-932.
Hammers, D. W., et al. (2019). Growth differentiation factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice. Scientific Reports, 9(1), 1-12.
Ouchi, N., et al. (2010). GDF11 inhibits angiogenesis and tumor growth in human melanoma cells. Oncotarget, 1(7), 1-9.
Kraler S. et. al. (2023). Circulating GDF11 exacerbates myocardial injury in mice and associates with increased infarct size in humans